Orthofix recognizes the importance of collecting data from centers around the globe as we continue to monitor the clinical performance and safety of the M6-C artificial cervical disc. Please see some of the key data points of this innovative non-fusion solution treatment in our interactive experience through the link below.
Patient Focused.
Proven Results.
Real World Evidence
70,000+ Devices Implanted
The M6-C artificial cervical disc is designed to maintain the natural behavior of a functional spinal unit by replicating the biomechanical characteristics of the native disc. The M6-C disc has established a robust clinical history with over 70,000 devices implanted in over 20 countries.

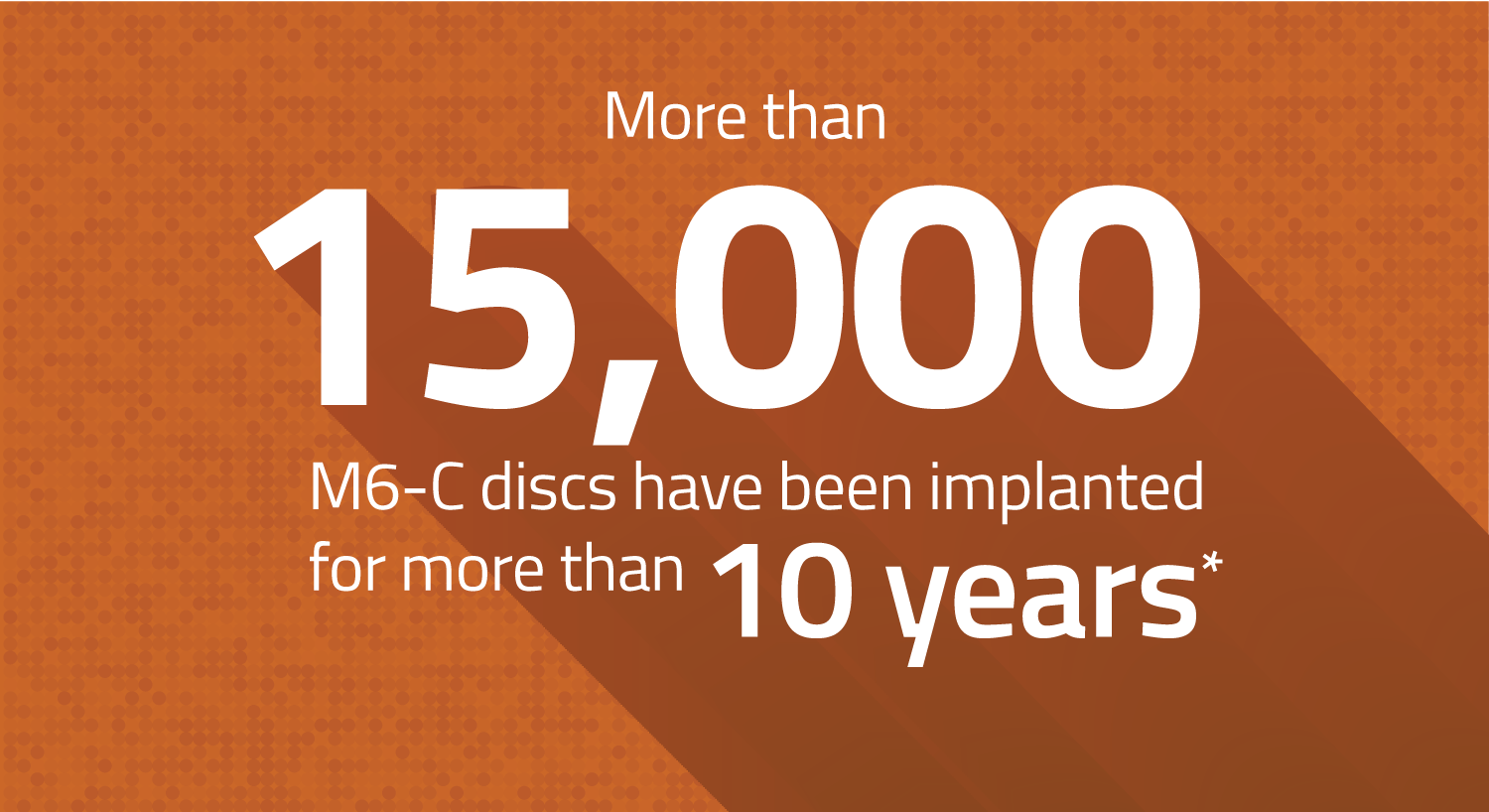
Over 15,000 Devices Have Been Implanted For More Than 10 Years
First introduced outside the U.S. in 2006, the M6-C disc has a significant clinical history. Of the 70,000 devices implanted globally:
- 40,000 discs have been implanted for more than 5 years
- 30,000 discs have been implanted for more than 7 years
- 15,000 discs have been implanted for more than 10 years

99% Device Survivorship at 10 Years
Results of a Kaplan Meier analysis of the M6-C™ artificial cervical disc based on 16 years of real world evidence suggest a global cumulative survivorship (percentage of implanted devices that are still intact and functional at a specific time period) of 99% at 10 years, consistent with other, proven, joint-arthroplasty devices such as hip and knee replacements.

Results from the U.S. IDE Study
At 5-years post-op, M6-C subjects had a mean NOi score of 8.0 – significantly better than the mean of 18.0 observed in the ACDF control group. At 7-years post-op, the M6-C subjects had a mean NDI score of 9.3. Therefore, the decrease in disability observed at earlier time periods is retained through 7-years post-op. Decreases in disability as measured by NDI and decreases in Neck and Arm Pain Scores that were observed at prior follow-up periods were retained through seven-years post-op.
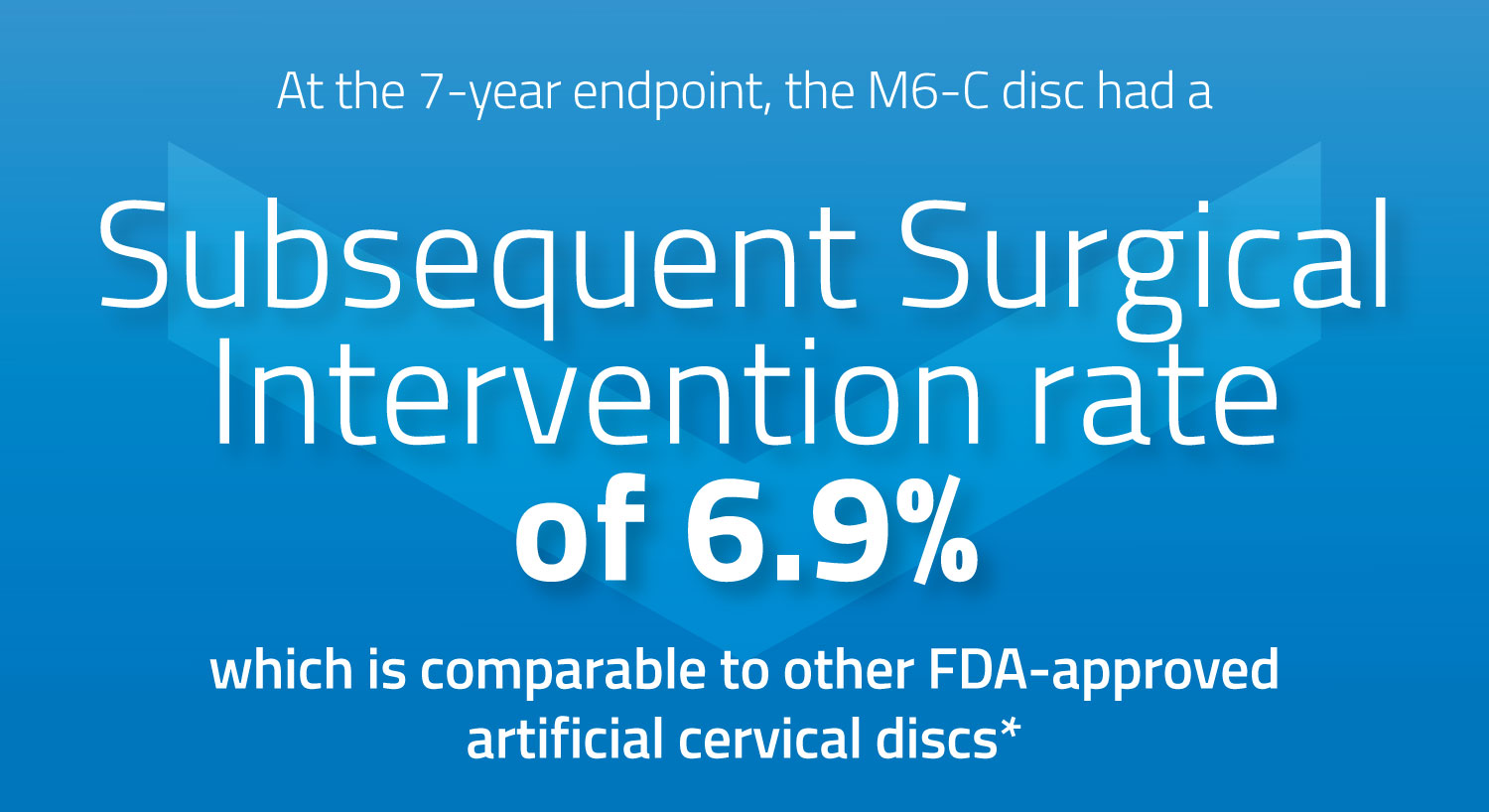
Subsequent Surgical Intervention (SSI) Rates at 7 Years
Eleven Secondary Surgical Interventions (SSI) were observed among the M6-C disc cohort (6.9%). This rate is comparable to seven-year SSI rates reported for other commercially available artificial cervical discs. Annual follow-up of this cohort will continue through 10-years post-op.
About the M6-C Artificial Cervical Disc
The M6-C artificial cervical disc is a next-generation intervertebral disc designed to restore physiologic motion to the spine and is indicated as an alternative to cervical fusion. The device is comprised of ultra-high molecular weight polyethylene fiber wrapped in a specific pattern, with multiple redundant layers that create a fiber matrix (artificial annulus). The fiber is then wound around a polycarbonate urethane polymer core creating an artificial nucleus. Like a natural disc, this unique construct allows for shock absorption at the implanted level and provides a controlled range of motion when the spine transitions in its combined complex movements. The M6-C artificial cervical disc is the only disc available in the U.S. with these features.