U.S. Clinical Study Results Shows Significant Improvement in Pain, Function and Quality of Life Scores, as well as Reduction in Pain and Opioid Medications Use.
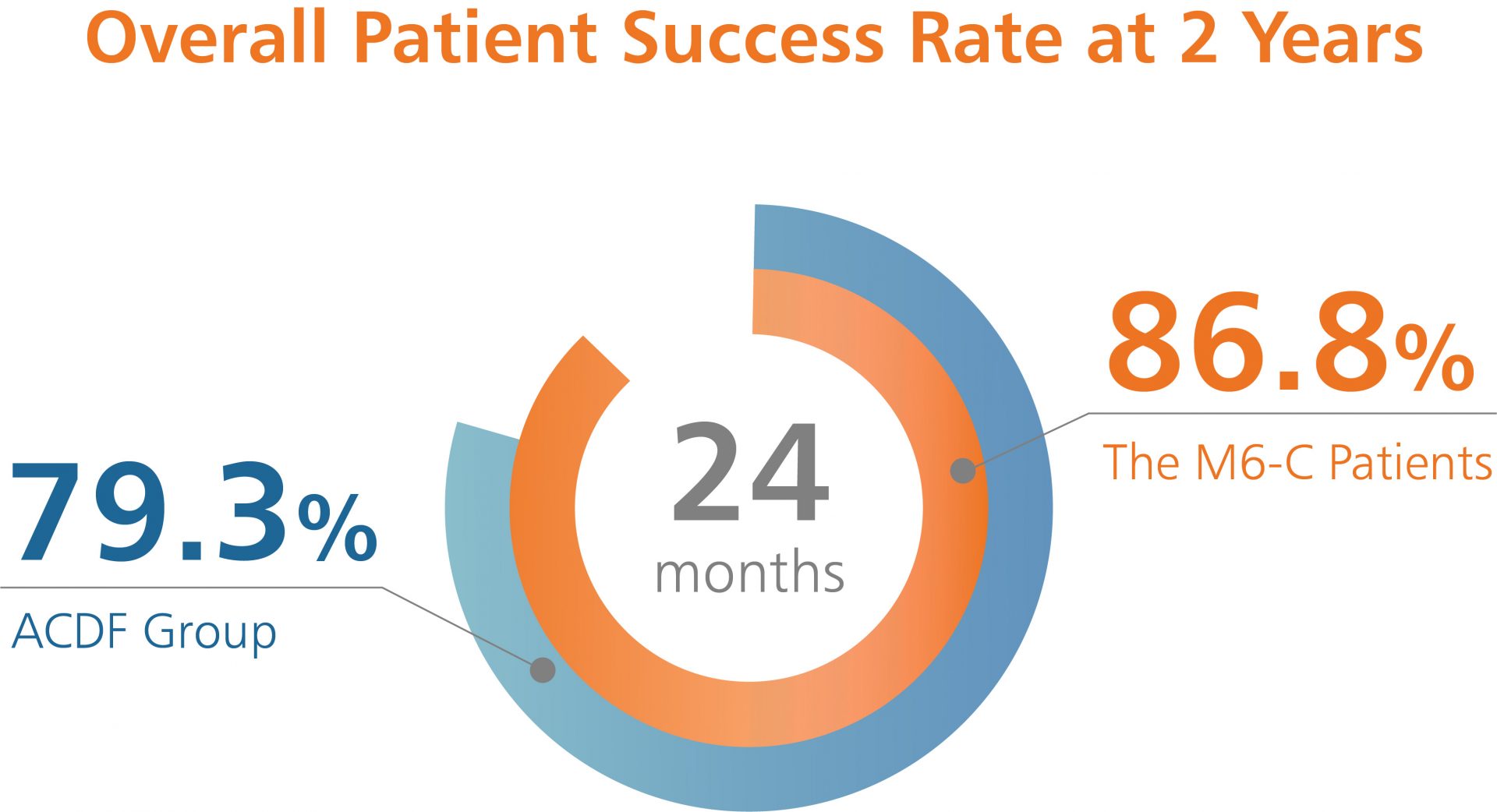
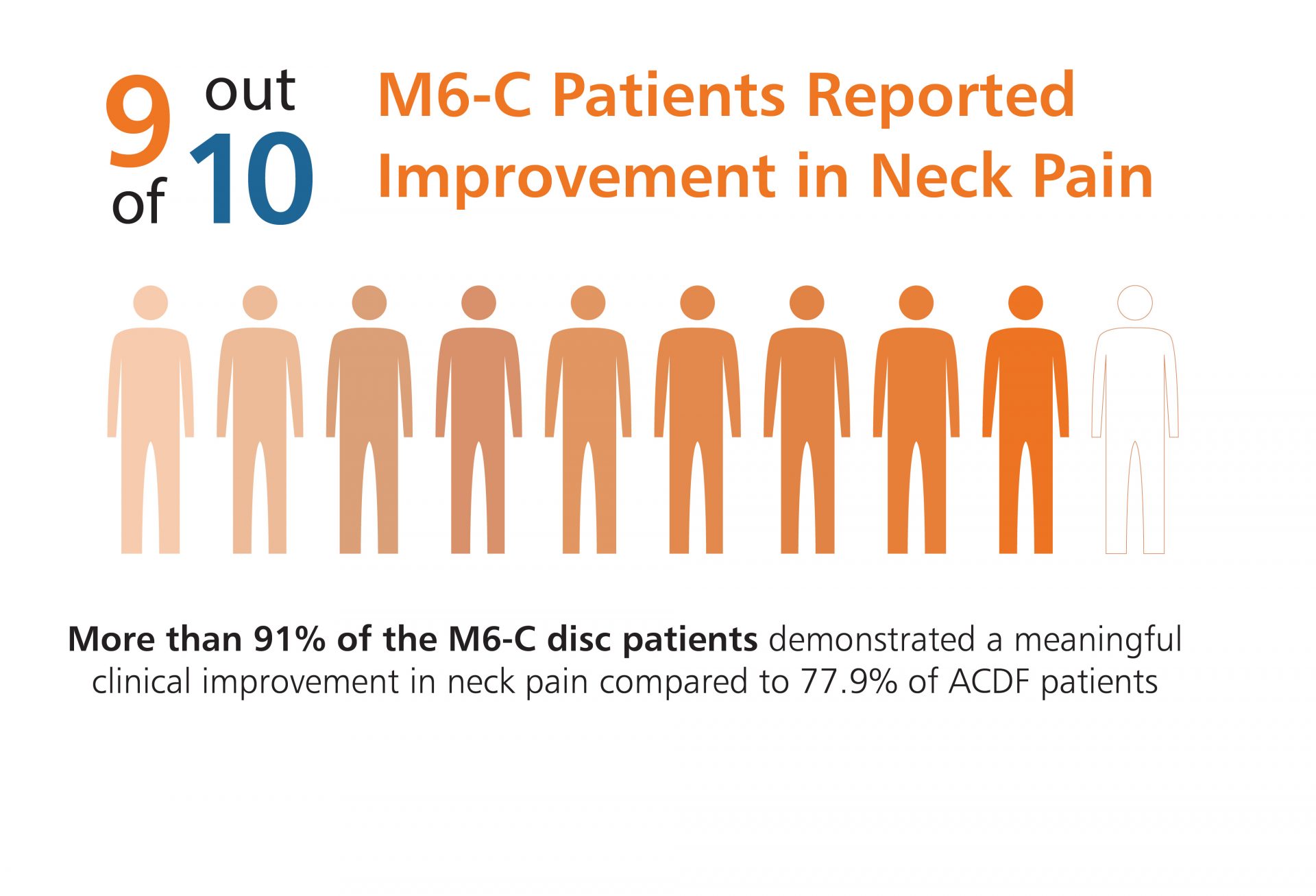
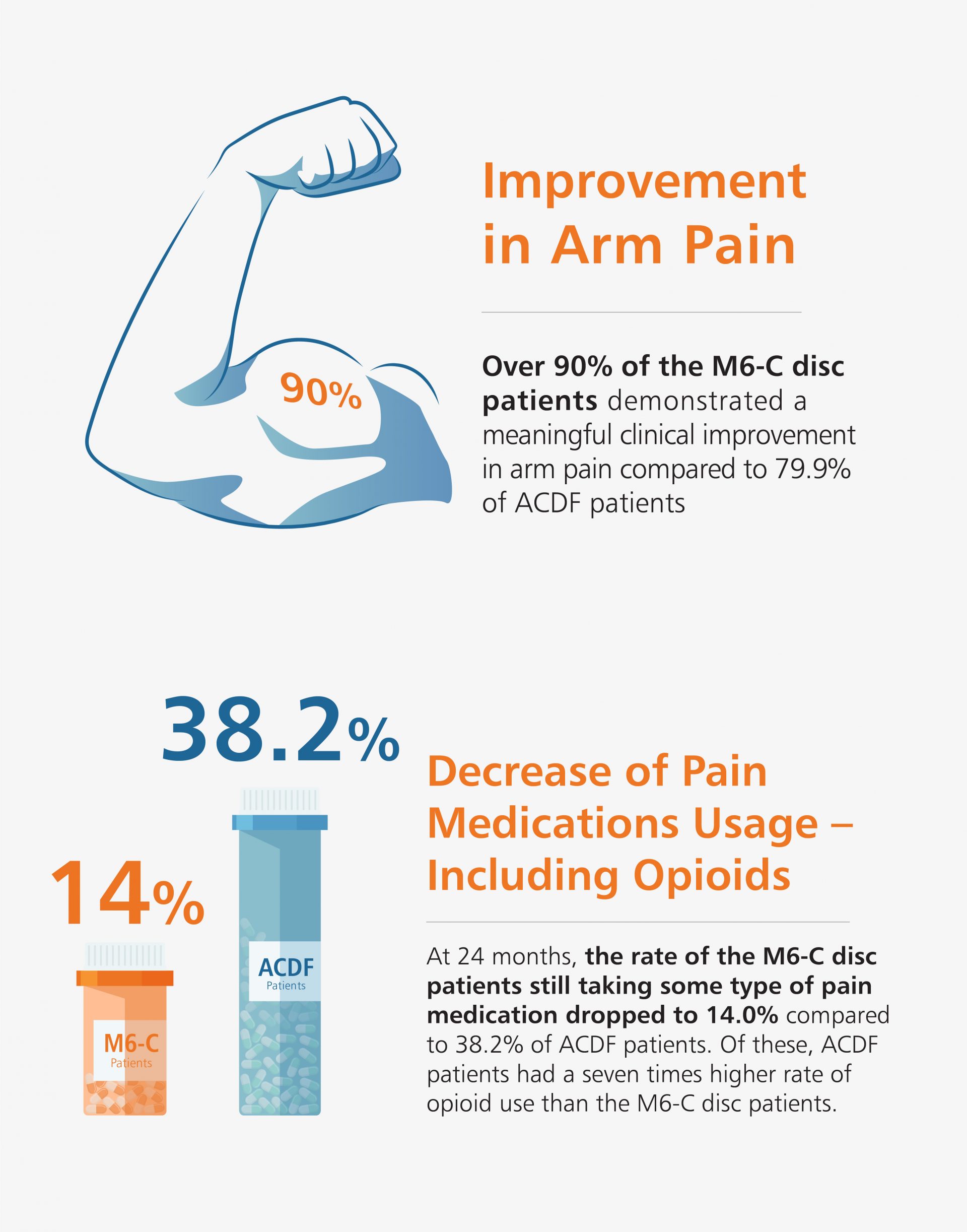
Like many new medical devices available in the U.S., the M6-C™ artificial cervical disc was evaluated through a large Investigational Device Exemption (IDE) clinical study to ensure the device was safe and effective prior to making it available to surgeons. The data from the IDE study was the basis for the U.S. Food and Drug Administration (FDA) approval of the M6-C artificial cervical disc for patients suffering from cervical disc degeneration. The U.S. study evaluated the safety and effectiveness of the M6-C artificial cervical disc by comparing it to anterior cervical discectomy and fusion (ACDF) for the treatment of symptomatic cervical radiculopathy with or without cord compression.