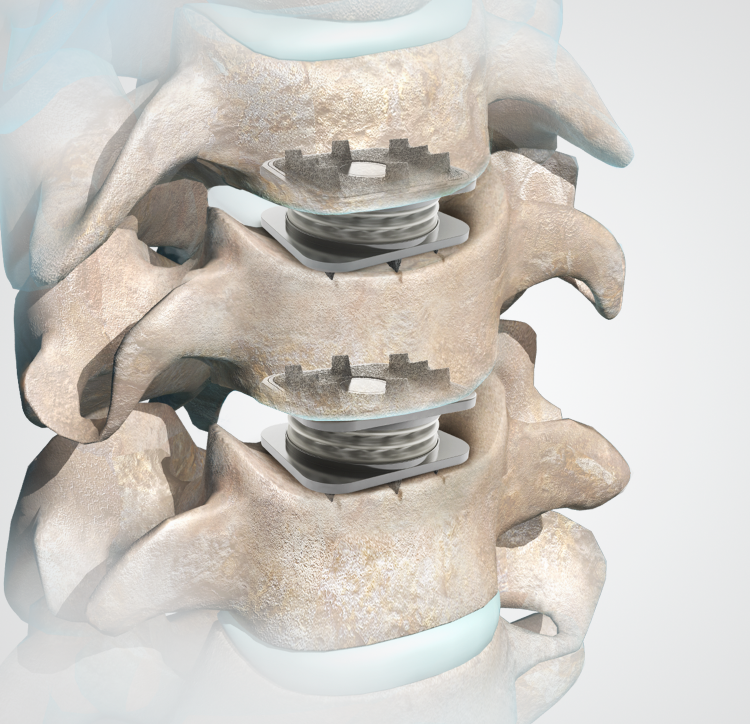
Orthofix announced the first patient implant in a U.S. Food and Drug Administration (FDA) clinical study that will evaluate the safety and effectiveness of the M6-C™ artificial cervical disc compared to anterior cervical discectomy and fusion (ACDF) for the treatment of contiguous two-level symptomatic cervical radiculopathy.
“Artificial cervical disc replacement is rapidly becoming the standard of care for patients suffering from degenerative cervical disc disease because it preserves motion, unlike ACDF procedures,” said Dr. Todd Lanman, a spinal neurosurgeon with Beverly Hills-based Lanman Spinal Neurosurgery and founder of the national ADR Advanced Disc Replacement Spinal Restoration Center who performed the first patient implant in the study. “The M6-C artificial cervical disc two-level study will provide additional data to validate the effectiveness of disc replacement over fusion in patients suffering from degeneration in two contiguous levels.”
Being conducted under a U.S. Investigational Device Exemption (IDE), the study will evaluate the safety and effectiveness of the M6-C artificial cervical disc compared to ACDF for the treatment of contiguous two-level symptomatic cervical radiculopathy at vertebral levels from C3 to C7 with or without spinal cord compression.To read the complete press release and learn more about the clinical study click here.